

OTS reduction process research and application
Wang Jianchun,Wang Xiangyu,Liu Rongxing
(Tangshan Outstanding Science & Technology Co., Ltd R&D center, Tangshan 063020)
Abstract: this paper briefly introduces the OTS process – the formation of car bottom furnace quickly reduction process, taking Indonesia laterite nickel ore and oolitic hematite for an example to make the research.
Key words: OTS process; Car bottom furnace; reduced iron. non blast furnace iron making
Introduction
China's current annual iron and steel production capacity has exceeded 700 million tons scale, and the structure of raw materials are in urgent need of improvement. In the newly introduced twelfth “five-year ”plan, non-blast furnace iron making technology is still one of the projects encouraged by the state government, under the guidance of national policies, non-blast furnace iron making technology has been concerned as the hot spot of the metallurgical industry in recent years. Direct reduction iron as the raw material for the production of high quality clean steel, in China's electric furnace steel in our country are in urgent need, which is also the urgent needed raw materials of China's equipment manufacturing high purity steel casting and forging billet production. Recently due to the rapid growth of iron and steel production capacity, the raw material problem is particularly prominent; the utilization of low-grade ore has become a hot spot of metallurgical industry. Coal-based direct reduction in processing low-grade ore, and using multi-metal mine, which has also made many attempts, the rotary hearth furnace, rotary kiln, tunnel kiln and furnace technology has many ways involved in the former item, but the several processes in treating low product beneficiation aspects of mine have failed to reach, many people in the industry is unsatisfied
It is in this background, our company’s relevant clients takes four years of hard study and experimental research, finally we introduced the OTS reduction process method, which is fast car bottom furnace reduction process system. The process is on the basis of traditional Hoganas tunnel kiln process, with the formation of a series of improvements and optimization, and its core equipment is the car bottom furnace, which is combined with a number of patented technology components. The most important characteristic feature of this process is the ability to maximize the recovery of low-grade refractory ore dressing, and ensuring a high metal rate. In this article, we selected a representative laterite nickel ore and Oolitic as an example, we also did a research and discussion for this process.
1 Test Method
1.1 Raw material preparation
We selected Indonesia laterite nickel ore and Korean Oolitic raw material as the oxide ore, the main chemical composition was shown in Table 1 and Table 2, the choice of the reduction coal was anthracite coal, with the particulate size of -100 meshes, the main ingredient in Table 3. this test is also used additives.
1.2 Test Equipment
The Reduction equipment is design by Tangshan outstanding science and Technology Co., Ltd. for OTS-8 car bottom furnace, its temperature measurement accuracy was around ± 5 ℃. In addition the test is also used XCGS-73 type ф50 magnetic separation tube and XMQ-ф240 × 90 cone ball mill and other equipment.
1.3 Test procedure
Adopting charge mold, we laid car surface layer about 10mm thick pulverized coal as basic material first, to prevent the material sticking car surface, the basic material is available for pulverized coal waste powders. And then put a reducing agent and a certain amount of mineral additives mixed uniformly on the car, the car be pushed into the furnace. After preheating, high temperature reduction, cooling and then exit the furnace. The reduced material on the car be put directly into the water for quenching. After cooling down the material will be crushed, milled and magnetic separated to get the DRI product.
2 test results
2.1 laterite nickel ore reduction
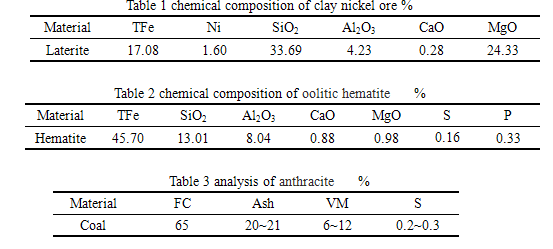
Through the repeated research and experiments, when reduction temperature was at 1300 ℃ ~ 1400 ℃, reducing time was 100 ~ 120min, using 1# recipes, which used car bottom furnace reduction combined with the magnetic separation technology, the product Nickel content is 15.7%, Ni recoveries is up to 99.22%.
2.2 Oolitic hematite reduction
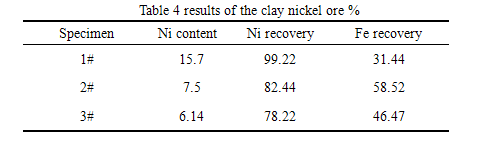
Using the 2 # recipe, through the test, we can concluded that the reduction temperature was at 1150 ℃ ~ 1250 ℃, the reduction time was under the condition of 100 ~ 120 min, using magnetic separation technology, the iron recovery rate is the highest, up to 92.10%, the total iron of iron concentrate was 90.50%, metallization rate was 97.01%.
3 reduction mechanism analysis
3.1 laterite nickel ore reduction mechanism
According to the reduction Gibbs free energy of nickel and iron oxide, we can know that the nickel and iron oxide can be easily reduced. Nickel oxide can be reduced at about 500℃. But the gangue oxide such as SiO2 and MgO can’t be easily reduced. Therefore, we can use the features of reduce nickel and iron oxide to control the reduction temperature, to make the gangue oxides not reduction, but nickel and iron have been reduced completely. Through the deeply reduction of Laterite nickel ore, ferronickel will be able to be separated from gangue components and other impurities. When using coal to reduce the laterite nickel ore, there are chemical reactions
C+CO2=2CO
△G=166550-171T, J·mol-1 (1)
NiO+C=Ni+CO↑
△G=134610-179.08T, J·mol-1 (2)
NiO+CO=Ni+CO2
△G=-40590-0.42T, J·mol-1 (3)
3Fe2O3+CO=2Fe3O4+CO2
△G=-52195.1-41.05T, J·mol-1 (4)
Fe3O4+CO=3FeO+CO2
△G=35120-41.55T, J·mol-1 (5)
FeO+CO= Fe+CO2
△G=-17500+21T, J·mol-1 (6)
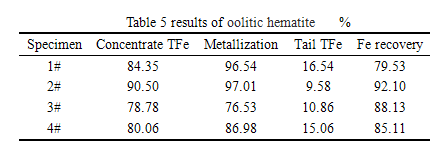
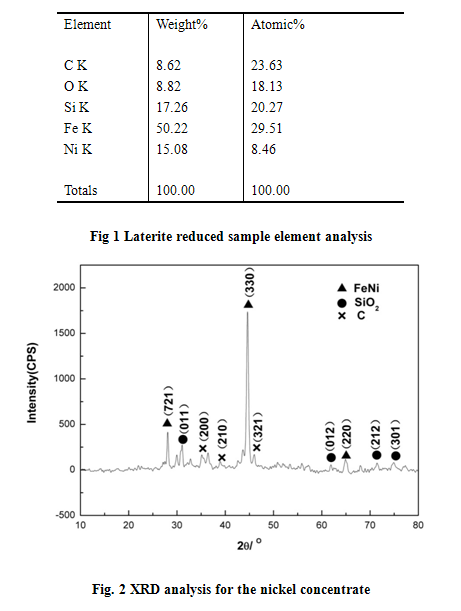
According to the XRD and EDS analysis for the nickel ore reduce and separated product, we can see that there are FeNi, SiO2, C phases in the concentrate. The result can be see in the following table and diagram.
3.2 Reduction Mechanism of Oolitic
According to iron and steel metallurgical theory, when temperatures above 1000 ℃, iron oxide reduction is mainly carried out step by step in the following order:
Fe2O3 →Fe3O4 →FeO →Fe
The iron oxide reduction reaction equation is (4)-(6), because of the lots of gangue and impurity such as SiO2 and Al2O3 in the oolitic, so during the reduction process, some fayalite will be generated.
2FeO + SiO2 = Fe2SiO4 (7)
FeO + Al2O3 = FeAl2O4 (8)
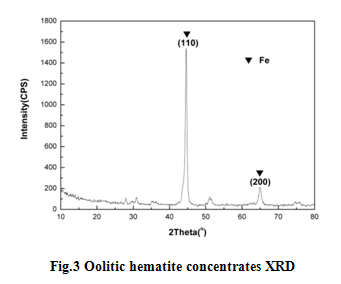
Through the XRD phase analysis of the concentrate reduction by magnetic separation oolitic hematite, the main phase of concentrates was Fe, as it is shown in Fig.3. This explains that the application of magnetic separation process combined OTS technology with maximize Oolitic iron recovery.
4. Conclusion
(1) Application of OTS technology combined with magnetic technology enables the reduction of nickel laterite ore processing, it is possible to achieve the recovery of nickel up to 95%. To achieve the recovery iron selectively.
(2) Using OTS process can achieve Oolitic reducing treatment, for the low-grade refractory ore dressing; using OTS technology can make iron recovery above 92%, metal ratio above 97%.
(3) because of its own characteristics, OTS technology in addition to achieve the laterite nickel ore and Oolitic reducing treatment, but is also capable for the difficult beneficiation of low grade processing. Ensure the metal ratio and recovery.
(This article was published in “2014 annual meeting of non-blast furnace iron making collected works”)